Medical Affair & Scientific Writing
At RCS, our Medical and Scientific Writing services ensure your research is presented with clarity and precision, meeting the highest standards of scientific communication
Our expert medical writing team offers flexible, end-to-end services, ensuring well-structured content that communicates effectively. With a comprehensive understanding of regulatory guidelines like ICH-GCP, FDA, and EMA, our qualified professionals develop regulatory documents and scientific publications across therapeutic areas, saving you time and costs. We support you from trial conceptualization to final approval, delivering CTD-compliant clinical submission documents.
17+ years of Medical Writing Experience
PhD Level Experts
>90% Medical Writers with Advance Degree
Broad Therapeutic Experience
20 years of Pre-Clinical and Clinical Research Experience
Excellence, reflected in every word we write
Clinical
Protocol Writing
Investigator Brochure Development
Informed Consent Document Development
Clinical Study Report (CSR) Writing
Regulatory Submission Summary Documents (IND/NDA/MAA/CTA)
Manuscript Writing
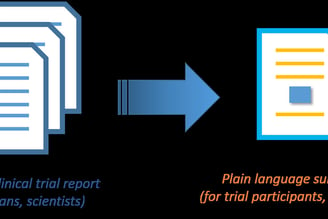
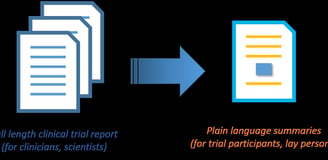
Plain Summary Report Writing
Our services
Safety
Development Safety Update Reports (DSURs)
Periodic Benefit Risk and Evaluation Reports (PBRERs)
Periodic Safety Update Reports (PSURs)
Periodic Adverse Drug Experience Reports (PADERs)
Risk Management Plans
Assessment of Benefit Risk (ABR)
Annual Reports
Patient Safety Narratives
Pre-Clinical
Pharmacology, Pharmacokinetic, and Toxicology Reports (in vivo/in vitro)
Bioanalytical Reports
Devices
Clinical Evaluation Plans (CEPs)
Clinical Evaluation Reports (CERs)
Investigational Device Exemption (IDE)
Publications
Scientific Manuscripts/Abstracts
Posters
Literature Reviews/Summaries
Oral Presentations
Other Scientific Documents
Plain Language Documents (protocol synopses, results summaries)
Informed Consent Forms (ICFs)
Regulatory White Papers
Consumer Health Product Documents








Benefits of RCS
World-class medical writing support led by doctors.
Single-vendor efficiency, simplifying project management.
Scalable resources to meet project demands.
Global multilingual content support for diverse markets.
100% regulatory compliance guaranteed.
Assured quality and timely deliveries.
Version control and harmonization to mitigate risks.
Streamlined, end-to-end solutions for reduced timelines and cost savings.
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de xira, Portugal
Madrid, Spain
Contact us:
+91-9820507220
+1(737) 313-4316
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
