The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137
Solution to the Challenges of Medical Writing
Medical writing is a specialized field that combines scientific knowledge with writing skills to produce clear, accurate, and accessible documents for various audiences, for example Clinical trial/study reports (CSR), Investigator brochures (IBs), Informed consent forms (ICFs), Manuscripts, Case reports, Blogs, Whitepapers, Regulatory documents, Promotional material etc. Despite its importance in disseminating medical information, medical writing comes with several unique challenges which can be overcome by partnering with RCS. Keep reading to find out more about how these challenges may hamper the quality of results and why RCS is your one-stop destination for all kinds of scientific and medical writing activities.
MEDICAL WRITING
2/20/20255 min read
Solution to the Challenges of Medical Writing
Medical writing is a specialized field that combines scientific knowledge with writing skills to produce clear, accurate, and accessible documents for various audiences, for example Clinical trial/study reports (CSR), Investigator brochures (IBs), Informed consent forms (ICFs), Manuscripts, Case reports, Blogs, Whitepapers, Regulatory documents, Promotional material etc. Despite its importance in disseminating medical information, medical writing comes with several unique challenges which can be overcome by partnering with RCS.
Keep reading to find out more about how these challenges may hamper the quality of results and why RCS is your one-stop destination for all kinds of scientific and medical writing activities.
a. Study Design & Scientific Validation
It is of utmost importance to bring scientific validation in study designs, protocols, endpoints and every single aspect of medical writing dynamics. Medical writing experts at RCS stress on thorough literature survey and scientific analysis before creating any study outline and/or protocols. The medical writers provide appropriate study design for the concerned protocol, which may translate into effective and reliable clinical trial data. It ensures that the study objectives, methodology, endpoints, inclusion/exclusion criteria, and other essential elements are standardized, reducing variability in how the research is conducted across different sites. We also ensure that the endpoints are aligned with the study objectives leading to more fulfilling and result-oriented research.
b. Complexity of Medical Terminology & Therapeutic Area Specialization
Medical writing often involves the use of complex medical terminology and jargons. It should be ensured that medical writing experts are acquainted with these terms and use them correctly. Besides, many writing professionals lack understanding and experience in niche therapeutic areas which turn into a major concern during study design and protocol development. At RCS we are glad to have professional medical writers who come with extensive experience and a deep understanding of the subject matter. To strengthen the subject knowledge we provide protocol training and/or therapeutic training. The targeted trainings help the subject-matter experts to stay tuned with the latest trends and core knowledge for complex projects. Our goal is to make readers and sponsors get the best support in terms of medical writing services.
c. Adapting to Audiences
Medical writers must tailor their writing style to suit different audiences, including healthcare professionals, pharma companies, patients, regulatory bodies, and the general masses. Each audience requires a different level of detail and complexity, making it necessary for writers to adapt to the demand and needs. At RCS our dedicated experts make sure that the writing produced is top-notch, reader-friendly, and as per the demand
d. Accuracy and Precision
Accuracy is paramount in medical writing because even a small error can have serious consequences. All it means is that the medical writers must be meticulous in their research, cross-referencing multiple sources, validating the source, and adhering to strict guidelines to ensure the information is both accurate and up-to-date. RCS medical writing team brings scientifically accurate reports, protocols, manuscripts, and other related documents without compromising on quality.
e. Regulatory Compliance
Medical documents, especially those related to clinical trials and drug development, must comply with various regulatory standards and guidelines. Understanding and keeping up with these regulations, which can vary by country and region, adds another layer of complexity to the writing process. Our team of dedicated experts help with medical writing projects which are fully aligned with the concerned regulatory landscape. RCS team remains updated on the latest guidelines and conducts regular training sessions on the updated rules and guidelines to the medical writers. Along with that, we make use of standardized templates and checklists which are aligned with current regulatory standards to ensure that the documentation is done maintaining all ethical standards.
f. Ethical Considerations
Medical writers must navigate numerous ethical considerations, such as ensuring patient confidentiality, avoiding plagiarism, and presenting unbiased information. They must balance these ethical concerns with the need to communicate effectively and transparently. Any error caused due to overseeing the details harms the entire procedure. RCS ensures that the documents we create are error-free, precise, and convey transparency. The medical writing professionals use advanced plagiarism-detection software for maintaining the documents’ originality. Additionally, they follow clear authorship guidelines to keep the documents well-structured, and ethics training programs for professional development.
g. Interdisciplinary Collaboration
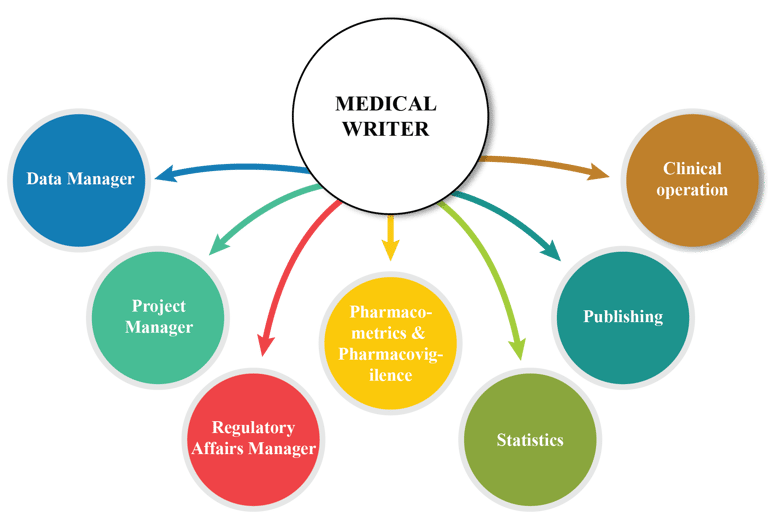
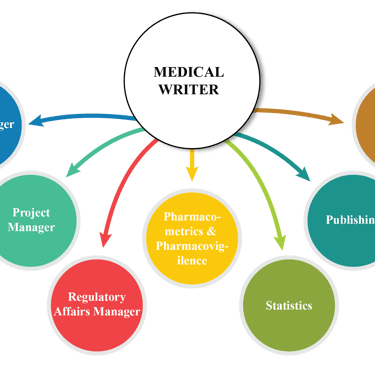
Medical writing often involves collaboration with healthcare professionals, researchers, regulatory authorities, and other stakeholders. Effective communication and collaboration are essential to gather accurate information and produce high-quality documents, but coordinating between different parties can be challenging. To overcome this challenge, RCS operates with medical writers coming with 10+ years of technical experience, efficient communication, and relation building experience. We provide seamless cross-party communication and project management.
h. Insufficient or Incomplete Information from Sponsors
Another major challenge is when the sponsor may miss sharing some important information regarding the study idea or provide incomplete data, unclear briefs, or deficient articles (e.g., incomplete clinical data, outdated medical device information, or irrelevant references). As a medical writer, it is imperative to understand the key components like objectives, endpoints, inclusion/exclusion criteria, patient population, interventions, and adverse reaction events that might occur during the study. In such critical situations the medical writers at RCS define clear study parameters, zero-in on the gaps and background knowledge by performing in-depth literature reviews to cross-check the missing dots, and develop a checklist of subsequent requirements to share it with the sponsor for a seamless project kickoff.
i. Statistical Analysis
The data produced during the clinical trial needs to be weighed effectively to avoid biasness and redundancy. RCS medical writers are well-trained with advanced statistical tools and programs for data analysis and validity. We also have a dedicated team of bio-statisticians to run in-depth analysis, cross-check the results, and overcome any error.
j. Client Expectations and Unexpected Document Revisions
What happens when the sponsor is sending the documents for revisions and there is no clarification on the next steps from the medical writer’s end, especially when there are multiple projects being undertaken simultaneously? Well, RCS gets you covered in that extreme situation as well! We develop style guides and templates for uniformity of documents, perform independent QC checks within the team before sending it to the sponsor. Even when amendments are necessary, the medical writing team is always prepared with a clear plan of action. We prioritize feedback, track changes, and focus on key sections to ensure a successful outcome.
k. Time Constraints
Tight deadlines are a common challenge in medical writing. Medical writing experts must produce high-quality documents be that Clinical Study Reports (CSR), Protocol and/or IB development, Manuscripts, etc. This pressure can be particularly intense in fast-paced environments like pharmaceutical companies or medical journals. In such situations, you can trust RCS as your medical writing and publication partner for timely delivery and publication in reputed, peer-reviewed journals. Our medical writing experts delegate the concerned tasks within the team following suitable strategies and provide quick resolution without compromising on accuracy and clarity.
Take home message: Experience hassle-free writing and publication services by executing your scientific and medical writing tasks via RCS. We take pride in associating with reputed pharma and nutraceutical companies on a global scale to assist them in important clinical research and scientific/medical writing projects.
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
