The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137
Scope of Medical Writing as Profession
Medical writing is a crucial discipline that acts as a bridge between complex clinical/medical research and the general masses, healthcare professionals, or regulatory authorities. It’s a field that requires a unique blend of scientific knowledge, medical terminology, and effective communication skills. Since medical writing involves creating accurate content which includes manuscripts/research papers, clinical study reports, regulatory documents, educational material etc., one of the key challenges is ensuring that the complex data is translated into documents without losing scientific integrity and the core concept. There are various forms of medical writing as required by the specific industry, each serving a different purpose and audience. Let’s find out what are those types and how you may train yourself to accommodate the skills required to work as a professional medical writer.
3/21/20253 min read
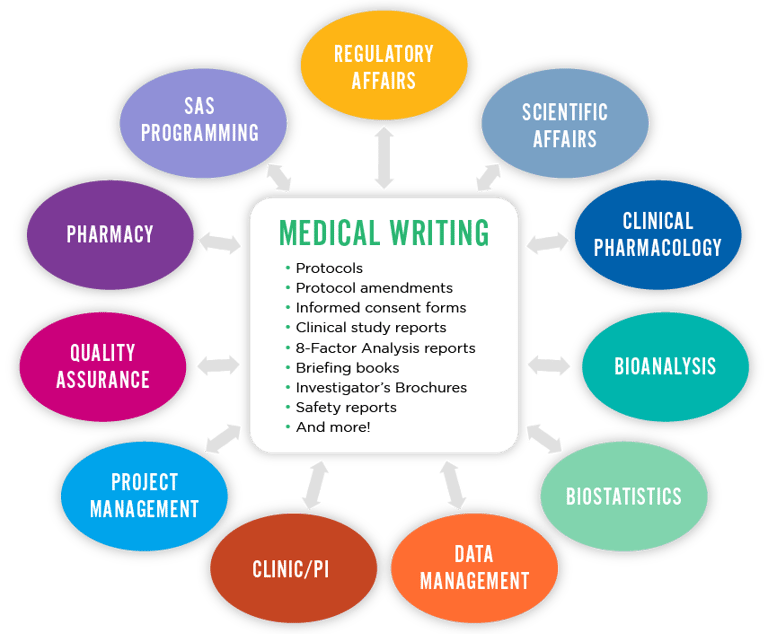
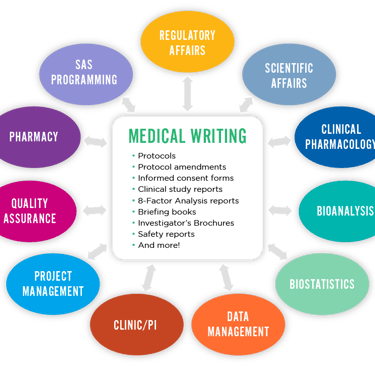
Why opt for Medical Writing as a Profession?
Medical writing is a crucial discipline that acts as a bridge between complex clinical/medical research and the general masses, healthcare professionals, or regulatory authorities. It’s a field that requires a unique blend of scientific knowledge, medical terminology, and effective communication skills.
Since medical writing involves creating accurate content which includes manuscripts/research papers, clinical study reports, regulatory documents, educational material etc., one of the key challenges is ensuring that the complex data is translated into documents without losing scientific integrity and the core concept.
There are various forms of medical writing as required by the specific industry, each serving a different purpose and audience. Let’s find out what are those types and how you may train yourself to accommodate the skills required to work as a professional medical writer.
Regulatory Writing: In this type of writing, the medical writer needs to create clinical study/trial reports and documents that are required for the approval of new drugs or medical devices.
Clinical Study Reports (CSRs): These are the detailed documents of clinical trials and their results, having in-depth and relevant information on the trial and its outcome.
Investigational New Drug (IND) Applications: This type of documents are submitted to the concerned regulatory agencies like the FDA, CDSCO, DCGI, IEC, to oversee the approval procedures for clinical trials.
Annual Reports: Summarizing safety and efficacy data for the ongoing products.
Scientific Writing: It involves writing about scientific research and discoveries, for example:
Original Research Articles: Research findings, investigations, protocol development, methodologies, results, validating the research findings, etc.
Review Articles: Collating the research conducted in a specific field, on a particular topic or disease, performing intensive literature survey, summarizing the existing research and finding the gaps in knowledge and defining future directions.
Abstracts: Short summaries of scientific studies provided in the beginning of the research article. Abstracts are also submitted for conference proceedings and/or publications.
Posters: Presentations of research findings in the form of large posters, generally used for presenting the research at conferences.
Publications: Finding suitable journals for publishing research articles, review articles, case reports, etc.
Pharmacovigilance Writing: This type of medical writing is concerned about the safety profile of drugs and medical devices. For example:
Adverse Event Reports: These are detailed reports about any negative effects of a device or drug on a patient.
Risk Management Plans: These documents provide strategies for minimizing the risks associated with a pharmaceutical product.
Educational Writing: In this type of writing, the medical writer creates material which aims at educating healthcare professionals or the public.
Continuing Medical Education (CME) Materials: CME refers to the educational content developed for healthcare industry to keep the professionals updated with advances in the medical field.
Patient Education Materials: Information designed to help patients understand medical conditions, treatments, and procedures.
Medical Marketing and Communications: This type includes writing materials aimed at promoting healthcare products or services. They may include sales and promotional copies, press releases, web content, social media posts, etc. The goal is to market a product or service effectively while adhering to ethical standards.
Medical Transcription: Transcription writing involves converting verbal medical reports (from doctors or healthcare professionals) into written form. It usually refers to accurate patient records and legal documentation.
Grant Writing: This type of writing involves creating proposals to grab funding for medical research, clinical trials, or public health initiatives from government bodies such as the NIH, ICMR, DBT, DST, SERB, etc.
Conclusion
Medical writing is a multifaceted field that combines expertise in medicine, science, and communication. The development of new drugs, therapies, and treatments, requires effective communication of this information to improve patient outcomes, assist in regulatory processes, and contribute to the broader understanding of medicine. With constant advancements in the above areas, medical writing has become an integral part of the healthcare industry and is a promising sector for professionals to choose as a career option.
Who can Become a Medical Writer?
To create a mark in the medical writing industry, one must come from a scientific and/or healthcare background and possess unique skill set that combines scientific expertise with strong research & development; excellent writing abilities is a plus.
Clinical Experience: Coming from a clinical research background is an added advantage because that may assist you in the medical writing role where the focus is on pre-clinical or clinical trials, and patient-centered documents like informed consent, investigational brochures, clinical study reports, etc.
Research Experience: A strong research background helps you in working out the protocols, methodologies, and preparation of manuscripts efficiently, further letting you transition into medical writing, especially scientific or regulatory writing.
Pharmaceuticals/Biotechnology Experience: Pharmaceutical or biotechnology industry experience is highly recommended, especially for regulatory writing or documents related to drug development.


Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
