The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137
RCS, your partner in Risk-Based Monitoring of Clinical Trials
In an era where precision, speed, and patient safety define success in clinical trials, Risk-Based Monitoring (RBM) has emerged as a game-changer. This strategic approach transforms how sponsors and CROs manage data integrity and trial oversight; moving from traditional, resource-heavy models to agile, intelligence-driven processes. Standing tall at the threshold of this transformation is RCS (RamAayanaM Clinical Solution), delivering next-generation RBM strategies that not only meet global regulatory expectations but consistently exceed sponsor goals.
5/13/20252 min read
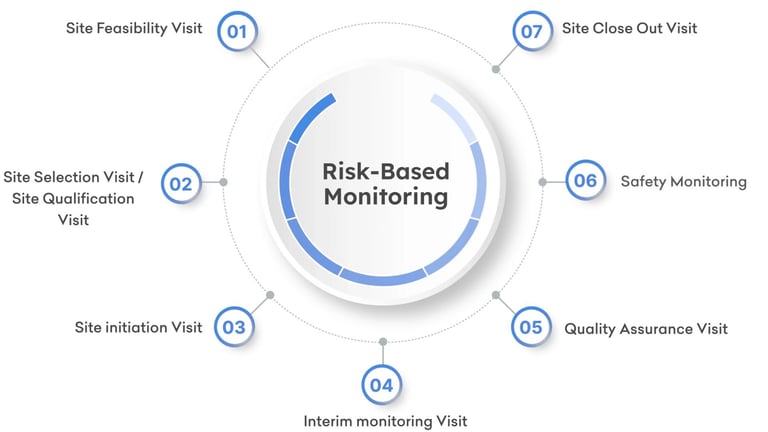
Here's how RCS stands out:
We provide tailored risk assessments: Every protocol is evaluated upfront to identify critical variables and tailor monitoring plans accordingly—ensuring that nothing vital slips through the cracks.
We extend support through centralized intelligence hub: RCS's centralized monitoring teams harness real-time data platforms to continuously oversee trial operations, proactively flagging issues before they become problems.
We are on-site with purpose: Our targeted on-site visits are informed by data, not tradition—delivering higher impact with fewer resources.
We come with multidisciplinary expertise: From medical monitors to data managers, RCS brings together the best minds to interpret signals and ensure holistic risk mitigation.
We offer regulatory confidence: With deep expertise in GCP and global regulatory frameworks, RCS ensures full compliance while keeping trials agile and audit-ready.
The Results Speak for Themselves!
Sponsors partnering with RCS consistently report:
30–50% reduction in monitoring costs
Significant improvements in protocol compliance
Faster issue resolution
High confidence from regulators and auditors
Takeaway
Risk-Based Monitoring isn’t the future—it’s the now! And RCS is already delivering results with it. Whether you're launching a global Phase III study or a targeted rare disease trial, RCS's RBM model ensures every decision is data-driven and every patient is protected.
Are you ready to elevate your clinical trial oversight?
Contact our team at +1 7373134316 or write us at info@rclinicalsolution.com to discover how RBM can redefine your success.


RCS, your partner in Risk-Based Monitoring of Clinical Trials
In an era where precision, speed, and patient safety define success in clinical trials, Risk-Based Monitoring (RBM) has emerged as a game-changer. This strategic approach transforms how sponsors and CROs manage data integrity and trial oversight; moving from traditional, resource-heavy models to agile, intelligence-driven processes.
Standing tall at the threshold of this transformation is RCS (RamAayanaM Clinical Solution), delivering next-generation RBM strategies that not only meet global regulatory expectations but consistently exceed sponsor goals.
What is Risk-Based Monitoring?
Risk-Based Monitoring is a proactive methodology that focuses monitoring efforts on the most critical aspects of a clinical trial—those that directly affect patient safety and data quality. As trials become more complex and global, traditional one-size-fits-all monitoring is no longer sustainable. RBM offers measurable advantages that make it the gold standard for modern clinical development. Instead of spreading resources equally across all sites and data points, RBM zeroes in on where risks are highest, using real-time analytics, centralized oversight, and adaptive processes.
Key Components of RBM
a) Centralized Monitoring – Real-time data review and analytics to identify anomalies, trends, and risks before they escalate.
b) Adaptive On-site Visits – Visits are targeted, data-driven, and prioritized for high-risk sites.
c) Continuous Feedback Loops – Seamless communication among sponsors, monitors, and investigators allows swift course correction.
d) Regulatory Alignment – Strategies are in line with FDA, EMA, and ICH GCP E6(R3) guidelines.
RCS Setting the Benchmark in Risk-Based Monitoring
A strategized RBM model provides improved data quality, enhanced patient safety, reduced monitoring costs, faster decision-making, agile and adaptive trial oversight, and many more such benefits. At RCS, Risk-Based Monitoring is considered as a core competency that drives smarter, safer, and more efficient clinical trials.
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
