The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137

Pharmaceutical Clinical Trials
The Journey of Healing: Unveiling the route out of the complex Pharmaceutical Clinical Trials
10/8/20235 min read
The path from discovery to medication is a profound journey marked by scientific inquiry, meticulous research, rigorous testing, and regulatory scrutiny. We at RamAayanaM Clinical Solution aim to embark on a journey that continues to shape the landscape of healthcare, offering promise and possibility to patients worldwide.
Innovations in clinical trials will lead to safer and more efficient clinical trials.
Why Do We Need Clinical Trials?
To assess the safety of new pharmaceutical compounds, monitoring for any adverse effects, side effects, and potential interactions with other medications.
To determine whether a drug or therapy is effective at treating the targeted medical condition. They also provide data on how well it performs in comparison to existing treatments.
To establish the right dose range for optimal therapeutic outcomes with minimal side effects.
To obtain regulatory approval for marketing a new drug, ensuring that it's safe and effective for patient use.
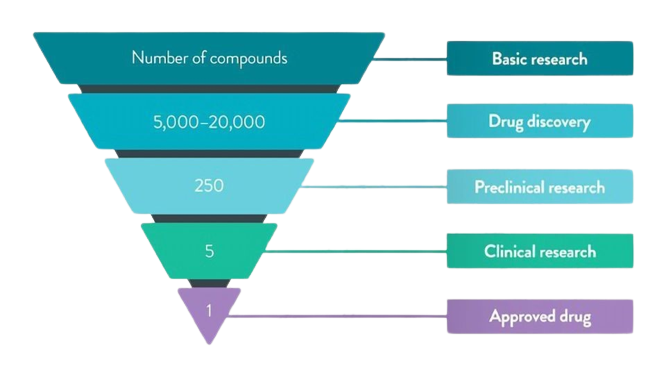
Drug development: A leaky pipeline
The industry has been flushing its resources into research & development and marketing. This is for its demand to expand and develop profitable margined products. From drug discovery through FDA approval, developing a new medicine takes at least 10 years on average and costs an average of $2.6 billion. Less than 12% of the candidate medicines that make it into Phase 1 clinical trials will be approved by the FDA.
In the realm of healthcare, the journey from scientific discovery to a safe, effective medication reaching patients' hands is an arduous and highly regulated process. We at RamAayanaM thrive on this epic quest to turn your promising compound into a life-changing treatment. Let us see how it happens!
The Seed of Hope: Discovery and Preclinical Research
Every new medication begins with a spark of inspiration, often in a research lab or the discovery of a potential target within the human body. The first step is to identify a molecule, protein, or pathway associated with a specific disease, and researchers screen countless compounds in search of a lead candidate. Once the lead is found, the compound undergoes preclinical research, where it's tested in the controlled environment of the laboratory and animal models. This stage aims to understand the drug's potential, including its safety, pharmacokinetics, and efficacy. Many promising leads never make it past this phase due to concerns about safety or efficacy.
The Gatekeeper: The Investigational New Drug (IND) Application
If a lead compound passes the preclinical tests, the pharmaceutical company submits an Investigational New Drug (IND) application to regulatory agencies like the FDA in the United States. This application includes preclinical data, proposed plans for clinical trials, and detailed information about the drug. The regulatory agency reviews the IND to ensure that the drug has the potential to benefit humans and poses minimal risks. Once approved, the IND serves as a passport to clinical trials, marking the beginning of the next stage.
The Human Touch: Clinical Trials
Clinical trials are the backbone of drug development. These trials involve a series of phases:
Phase I: Small-scale studies that evaluate the drug's safety, dosage, and pharmacokinetics in a small group of healthy volunteers.
Phase II: A larger group of patients with the target condition participate to assess both safety and efficacy. Some comparative data with existing treatments may also be collected.
Phase III: Large-scale trials to further evaluate safety and efficacy. These are typically randomized, controlled studies that provide the data needed for regulatory approval.
If the Phase III trials are successful, the pharmaceutical company submits a New Drug Application (NDA) to the regulatory agency, which includes all relevant data and proposed product labelling.
The Judgment Day: Regulatory Review and Approval
The regulatory agency conducts a rigorous review of the NDA, scrutinizing every aspect of the drug's development, safety, and efficacy. If the benefits outweigh the risks, and the drug gets the regulatory agency's approval, it can be marketed and made available to the public.
How can you save your Clinical Trials from failing?
A high percentage of drug candidates fail to reach the market during clinical trials. These failures have massive economic implications. Before initiating the trial processes, as a trial partner, it is essential to factor in challenges like chaotic and slow patient recruitment, lack of experience in choosing and monitoring partners, lack of feasibility of the study protocol, low quality of the registered data, too high incidence of serious adverse events and severe incidents, unmanageable level of portfolio complexity, and incorrect assessment of the market potential or returns cause the failure of clinical trials. It is essential to do accurate due diligence in choosing sites and subcontractors. Followed by which there should be careful monitoring. Sites should be encouraged to develop innovative techniques to facilitate patient recruitment (For e.g.: e-recruitment tactics). It would be beneficial for the future of the product to employ interim analyses. It can never be late for learning by failing mind-set. While developing the protocol, ensure that the testing procedures are adequately elaborated. In addition to it, give robust training in handling portfolio complexity and handling incidents and severe adverse events. At the end of the trial it would be resourceful to collect regular feedback from the sites.
What do these trials serve?
Pharmaceutical clinical trials are the heart and soul of modern medicine. These trials are an essential step in the process of bringing life-changing medicines to patients around the world. These cover a wide range of therapeutic areas, addressing various medical conditions and diseases. The choice of therapeutic area for a clinical trial depends on factors such as the specific drug or treatment under investigation, the needs of the patient population, and the goals of the pharmaceutical company or research institution.
Clinical trials in oncology focus on cancer treatments, including chemotherapy, immunotherapy, targeted therapies, and radiation therapy. Trials may aim to develop new cancer drugs or improve existing treatments for various types of cancer. Furthermore, trials also investigate drugs for conditions like hypertension, heart failure, diabetes, atherosclerosis, arrhythmias, Alzheimer's disease, Parkinson's disease, etc. They may aim to slow disease progression or manage symptoms. Based on recent surges and demands, clinical trials also propagate the development of vaccines, antibiotics, and antiviral medications for diseases such as HIV, tuberculosis, hepatitis, and emerging infectious diseases. Some trials investigate treatments for autoimmune diseases like rheumatoid arthritis, lupus, and inflammatory bowel diseases such as Crohn's disease and ulcerative colitis. In case of respiratory conditions, the trials focus on treatments for asthma, chronic obstructive pulmonary disease (COPD), and pulmonary hypertension. Clinical trials even address mental health conditions like depression, anxiety, schizophrenia, and bipolar disorder. They aim to develop new therapies or improve existing psychiatric medications. Pharmaceutical trials in dermatology concentrate on skin conditions like psoriasis, eczema, acne, and skin cancer. Researchers work on developing topical treatments and systemic medications. Additionally, Clinical trials investigate treatments for digestive system disorders, blood-related disorders, eye conditions, pain management etc. Clinical trials for children focus on paediatric-specific conditions, including paediatric cancers, congenital disorders, and infectious diseases, to ensure that therapies are safe and effective for young patients. Orphan drug trials concentrate on medications for rare diseases that affect a small percentage of the population. Regulatory incentives often support research in this area. Clinical trials in women's health focus on conditions like breast cancer, endometriosis, and menopause, addressing specific healthcare needs. Its contribution in Men’s health explores treatments for conditions such as erectile dysfunction, prostate disorders, and male reproductive health issues.
The scientific investigations are designed in a controlled and systematic manner, following a series of well-defined stages, all aimed at ensuring the development of safe and effective treatments for a variety of medical conditions.
We are ready to set the cornerstone of medical innovation and the gateway to ground-breaking advancements in healthcare. We are here to empower new treatments discovery, improve existing ones, and ensure that patients receive safe, effective therapies.
The dedication and rigor of clinical trial researchers, along with the participation of courageous volunteers, drive forward our understanding of disease, ultimately improving the lives of countless individuals. As we continue to make strides in medical research, pharmaceutical clinical trials remain the bedrock upon which we aim to build a healthier future for all.
Leave a Reply
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
