The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137

Nutraceuticals Clinical Trials
Unveiling the Science Behind Nutrition: Supplement your health with functional products!
10/8/20236 min read
The path to wellness is paved with good intentions, a balanced diet, and a sprinkle of nutraceuticals. RamAayanaM Clinical Solution lends support at every step of your clinical trial journey. We are here to help you carve a niche in the nutraceutical market allowing you to grow your market size and facilitate product differentiation. Our team of experts will help understand your requirements and provide solutions in accordance with regulatory requirements, right from the study design to commercialization. We at RamAayanaM Clinical Solution conduct our clinical trials to lead the way for new discoveries and innovations with quality. As your clinical trial partner, we also are bound to monitor the adherence of the clinical trials and related activities towards the concerned regulatory rules and guidelines. At RamAayanaM Clinical Solution, we are also equipped with high technology, advanced software, and top-notch scientific tools to make sure that we are constantly able to provide our clientele with accuracy, consistency, and quality.
The combination of nutrient and biologically active compounds along with its concerted mechanism of action, make them indicators of having a “possible beneficial role” for health. Unlike drugs, dietary supplements intended for the purposes of affecting the structure and function of the human body are not subject to approval from DCGI, FDA, or any other regulatory bodies, under the Dietary Health Supplement and Health Education Act of 1994 (DSHEA). However, the manufacturers of dietary supplements must ensure that their products are manufactured in accordance with Good Manufacturing Practice (GMP) guidelines, are labeled accurately, and are safe and free of contaminants. The European Union (EU) regulations for supplements also support health benefits and not therapeutic or medicinal claims. However, if the dietary supplement is intended to be used as a drug to diagnose, cure, mitigate, prevent, or treat a disease an IND is required to conduct clinical investigations in humans.
Nutraceuticals add an element of "nutrition" into the "pharmaceutical." They are naturally occurring compounds or products that provide health benefits, beyond basic nutritional value for their bioactive components. These include vitamins, minerals, herbs, antioxidants, and other dietary supplements, all of which are believed to aid in preventing or treating various health conditions. But how do we know if they can truly deliver on their promises?
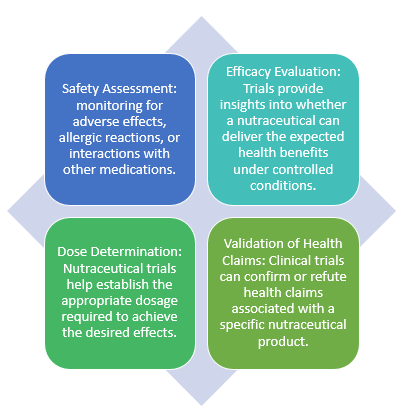
The key reasons for conducting clinical trials on nutraceuticals include:
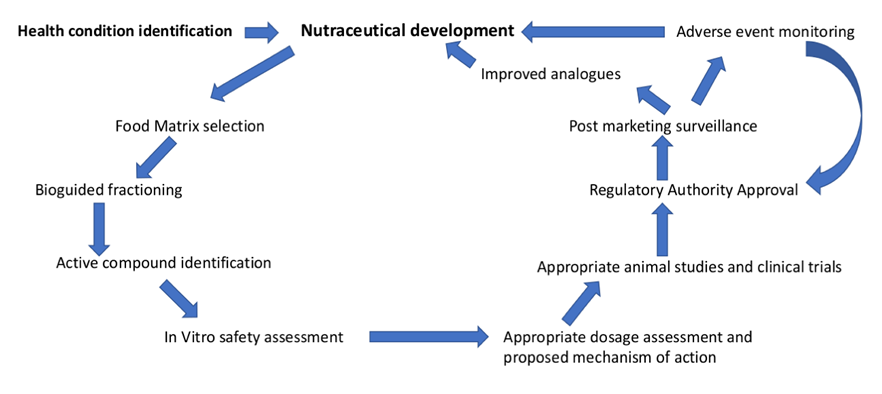
Nutraceutical clinical trials follow a structured process. For new and unexplored products, safety and pharmacokinetic assessments are conducted initially to gather data on its absorption, distribution, metabolism, and excretion (ADME) within the body. In this phase, a small number of healthy volunteers may be exposed to sub-therapeutic doses of the nutraceutical. Once the bioavailability is established, companies move on to conduct safety trials. These trials involve a small group of healthy volunteers or individuals with the condition of interest to understand the product's safety and dosing. Post which, efficacy trials are conducted on a large scale to gather data on the product's effectiveness, and optimal dosage and support claims about the nutraceutical's health benefits. Once these trials are completed, post-marketing surveillance is conducted which involves ongoing monitoring of the product's safety and efficacy in real-world use. Researchers and regulatory agencies continue to collect data on the product's performance and any adverse effects. All nutraceuticals will not necessarily go through all of these phases. The extent of clinical testing may vary depending on the specific product, its intended use, and the regulatory requirements in a given country. For products with already analyzed active ingredients are seldom analysed for large scale trials without the preclinical analysis depending on the pre-established data.
Clinical research can unlock incredible market opportunities for dietary supplements. Companies use clinical trial data to gain access to new markets, support unique claims or explore new regulatory pathways. The category of Nutraceuticals is dependent on the country's regulatory framework. For example, in Canada nutraceuticals are a part of the natural health products (NHPs) and supplemented foods. The European Food Safety Authority (EFSA), US Food and Drug Administration (FDA), and Health Canada take into account evidence-based reviews. These are evaluated to weigh the scientific evidence across the marketing claims of the prospective foods and dietary supplements. The growth of this industry is profoundly modulated by the principle of evidence-based medicine (EBM). It supports the ideology of an intervention that mitigates or attenuates a condition in a causal manner. The decades of piled evidence for such products facilitates the decision-making process. In order to bring your product to the spotlight, Randomized Controlled Trial is the key! It paves the way between intervention and its outcome. This is a pivotal element for the regulatory processes involved in approval of drugs and health claims of nutraceuticals. Thus, RCTs are an impeccable hallmark for evaluation of nutraceuticals and strengthen our understanding of these natural health enhancers.
It's important to note that while nutraceuticals can provide potential health benefits, but not all their claims are supported by robust scientific evidence. Hence clinical trials are conducted. These trials typically focus on various health indications, aiming to provide scientific evidence for the effectiveness of nutraceuticals in preventing, managing, or treating specific health conditions.
The goals of the clinical trials in this sector are either focussed on managing the symptoms, delay the disease progression, mitigate the risk or a potential aid for physiological, biochemical and structural changes. For instance, certain trials often explore the role of an ingredient in maintaining cardiovascular health. This can involve assessing their impact on cholesterol levels, blood pressure, and reducing the risk of heart disease. Ingredients like glucosamine, chondroitin, calcium and vitamin D are studied for their potential in improving joint health, especially for conditions like osteoarthritis, osteoporosis etc. Multi-strain Probiotics, prebiotics and symbiotic are commonly tested to determine their effects on gut health, including the prevention or management of digestive disorders like irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Furthermore, as immune enhancers supplement like vitamins, minerals, curcumin and antioxidants are examined for their ability to reduce the risk of infections. Some trials even focus on the impact of nutraceuticals on cognitive health and the prevention of conditions like Alzheimer's disease or age-related cognitive decline. Several studies are conducted to evaluate the potential of quercetin, rutin, etc. for supporting weight loss, control appetite, blood sugar control, the management of diabetes, metabolic dysfunction and manage obesity-related conditions. Additionally, supplements enriched with collagen, hyaluronic acid etc. are investigated for their effects on skin health, including their ability to reduce the signs of aging or improve conditions like acne. Several companies even claim their product as lifestyle supplements. Those are often tested for their potential in reducing stress, anxiety, and improving mood in people having disturbed or highly stressed lifestyle. A few supplements like lutein and zeaxanthin are booming for their role in maintaining eye health and reducing the risk of age-related macular degeneration. These supplements have also been found helpful in attenuating dry eye related symptoms in individuals who are into digital sports or are professional e-gamers. Additionally, several nutraceuticals are explored for their potential to support liver function and protect against liver diseases like NAFLD. Some trials even investigate nutraceuticals' role in reducing the risk of cancer or as complementary therapies for cancer patients. Some products claim restoring the electrolytes balance, aid dehydration symptoms and nausea in people undergoing chemotherapy. Nutraceuticals may be tested for their impact on metabolic conditions such as metabolic syndrome and insulin resistance. Nutraceuticals like quercetin may be studied for their effects on reducing allergy symptoms and improving respiratory health. Nutraceuticals have been supportive for many more such therapeutics like Benign prostatic hyperplasia, Menopausal symptoms, etc.
What can challenge your product?
Nutraceuticals often contain a combination of active compounds, making it difficult to isolate the specific ingredient responsible for the health benefit. Issues like variability in product quality, potential contamination, and a lack of funding for trials can hinder the research process. As a part of trial design, it is essential to define an appropriate baseline for the results to reach a statistical significance and control the placebo response. The endpoints in the study may not always align the product’s actual MOA at pilot stage. Hence the endpoints must be global or multifunctional while designing the study. Finally, the requirement for ‘healthy' participant enrolment can be challenging. Therefore, N-of-1 studies design is better suited for nutraceuticals where two interventions are compared with one another. It provides greater strength and more reliable data in clinical decision-making. Further, this design may provide specificity and inherently address the heterogeneity of treatment effects.
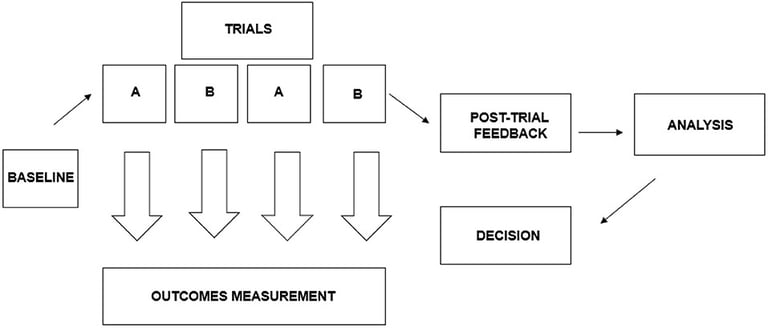
Nutraceuticals are a challenge to be the new mode of preference for prevention and therapy in the future. Adequate measures are needed in order to improve their bioavailability and elucidate that exact their mechanism of action. Several industries are adopting nanotechnologies as new delivery approach as opposed to the conventional ones. There lays another fantastic scope for clinical studies to assess their efficacy in improving the bioavailability.
As the field of nutraceutical research continues to evolve, the results of clinical trials will shape our understanding of these promising compounds and contribute to a more holistic approach to healthcare, where the best of nature and science combine to promote well-being sustainable environmental friendly solutions.
Leave a Reply
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
