The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137

Medical Devices Clinical Trials
Innovating Patient Care: The Vital Role of Clinical Trials in developing the pipeline for Medical Devices
10/8/20237 min read
In the ever-evolving landscape of healthcare, medical devices play a pivotal role in diagnosing, treating, and managing a wide range of medical conditions. These serve as the unsung heroes, empowering healthcare professionals and transforming patient care. Behind these ingenious devices is a journey that involves innovation, meticulous testing, and a commitment to enhancing human well-being. RamAayanaM Clinical Solution aims to be a part in your journey to bridge the gap between your device to the market.
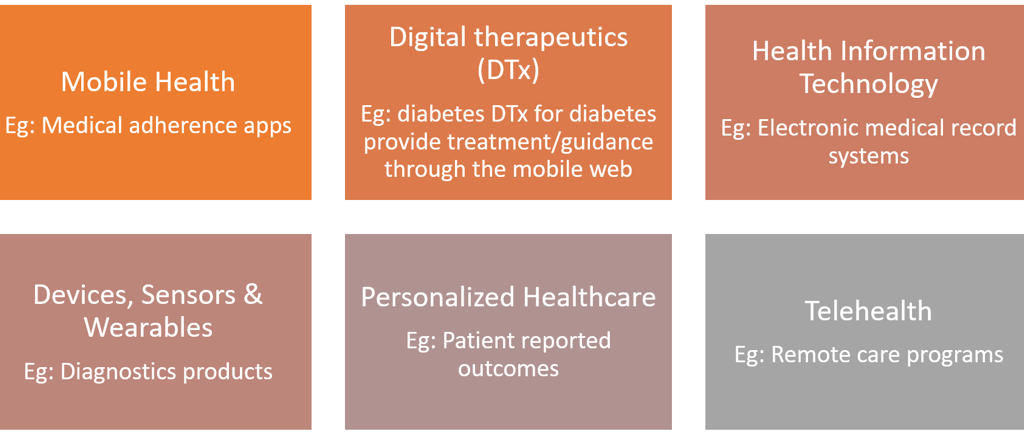
The era of digital therapy has been a hot topic as the new therapeutic category over the last few years. These deliver evidence-based therapeutic interventions to patients that are driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes. The products under this category incorporate advanced technology best practices relating to design, clinical validation, usability, and data security. They are reviewed and cleared or approved by regulatory bodies as required to support product claims regarding risk, efficacy, and intended use.
Most clinical trials of drugs at the site-level require an investigating physician, one or more clinical research coordinators or nurses, pathology staff, and third-party vendors. In comparison, medical device trials additionally require specifically qualified technicians, programmers, and medical imaging staff, as well as psychologists or physiotherapists, depending on the device.
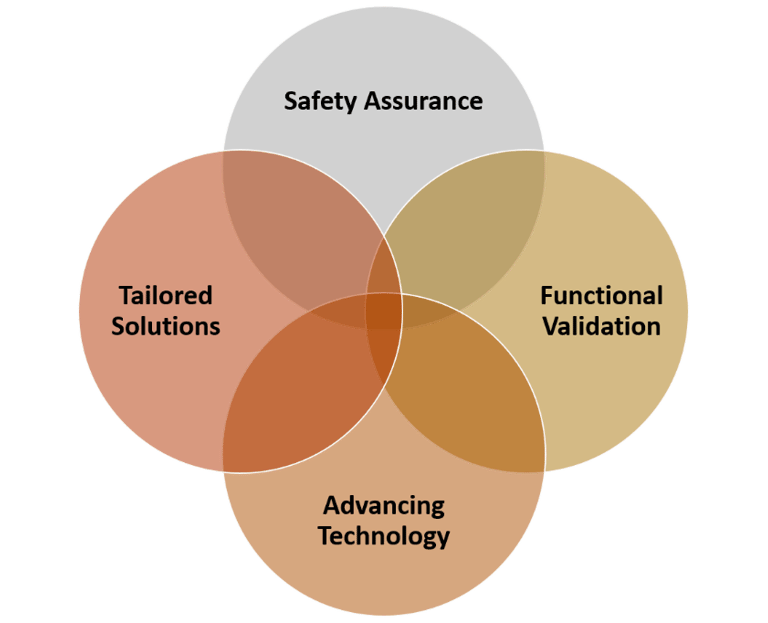
Medical devices range from diagnostic tools and imaging equipment to life-sustaining implants and innovative assistive technologies. Clinical trials for these devices play a pivotal role which, in turn, impacts the healthcare landscape in the diverse ways. They contribute in (i) ensuring safety (ii) designing devices specific to the indication (iii) Validation in different population and (iv) using advanced technology based on real world data.
The clinical trial process for medical devices typically encompasses several phases: Feasibility, Preclinical, Investigational Device Exemption (IDE) Study and Clinical Trials. The initial feasibility studies determine whether a device concept is viable and uncover potential issues that need to be addressed. This stage often involves testing in controlled laboratory settings. Next, the preclinical studies assess the safety and functionality of medical devices using animal models. They help researchers identify any potential risks or complications that can be foreseen for human trials. Before a medical device can be tested in humans, the sponsor often needs to obtain an IDE from regulatory agencies. The IDE outlines the clinical investigation plan, including study design, objectives, and patient population. Post these, comes the phase of conducting clinical trials. Medical device clinical trials typically follow a similar structure to pharmaceutical trials, including Phase I, Phase II, and Phase III studies, which focus on safety, efficacy, and large-scale validation. These studies involve human participants and provide data for regulatory approval.
In recent years, innovative intraoperative in vivo breast tumor diagnostic devices have been proposed to improve the accuracy and surgical outcomes of breast tumor patients undergoing resection. Although such technologies are promising, investigators need to obtain statistical evidence for the effectiveness and safety of these devices by conducting valid clinical trials. Clinical trials for medical devices cover a wide range of health indications, addressing various medical conditions and healthcare needs. Therefore, study design and statistical analysis are crucial in clinical trials to evaluate the effectiveness and safety of new medical devices under investigation. A very essential underrepresentation fact is that differences in demographics can influence the safety and effectiveness of medical devices. The US Food and Drug Administration (FDA) Safety and Innovation Act encourages greater diversity and subgroup analyses. In 2013, the FDA reported that there was diversity in clinical trials considered “pivotal” for approval decisions and that subgroup analyses were conducted for most applications for the highest‐risk medical devices. However, the FDA's report did not specify whether analyses included sufficient numbers to be meaningful, whether analyses were conducted for most major subgroups, or whether analyses included safety, effectiveness, or accuracy. Therefore, it is mandatory to factor in multicentre validation studies for medical devices that have been approved in one country to determine its safety across patient variability. The choice of health indications for a medical device clinical trial depends on the type and purpose of the device, as well as the specific patient population it aims to serve. For e.g.: Stents and angioplasty devices for the treatment of coronary artery disease; Implantable cardioverter-defibrillators (ICDs) and pacemakers for arrhythmia management; Cardiac monitoring devices for detecting and managing heart conditions etc. For Orthopedic and Musculoskeletal Conditions, Joint prostheses (e.g., hip and knee replacements) for improving mobility, Spinal implants and devices for the treatment of back and spine-related conditions, External and internal fixation devices for fracture management etc. are being evaluated as upcoming medical devices. Similarly, for Dental and Oral Health, Dental implants, Orthodontic devices for teeth alignment and oral diagnostic tools for detecting oral diseases and conditions are being evaluated. Devices like Intraocular lenses and cataract surgery devices are being evaluated for the ability in improving vision. Additionally, Glaucoma drainage devices, Retinal implants and prostheses are also being investigated. Under the indication of Respiratory Health, medical devices like Mechanical ventilators, Continuous positive airway pressure (CPAP), Nebulizers and inhalers are categorized. Dialysis devices, Ureteral and urethral stents are being explored for their potential in kidney disease management functions. Furthermore, the role of clinical trials in medical devices have also aided physicians in areas of gastrointestinal diseases (Gastric bands and intra-gastric balloons, Endoscopic and minimally invasive devices, Enteral feeding devices), reproductive health (Intrauterine devices (IUDs), Fertility monitoring and assisted reproductive technology (ART) devices), dermatology (Dermatoscopes and imaging devices), ENTs (Hearing aids and cochlear implants), Patient Monitoring (Vital sign monitors, ECG devices, MRI, CT etc.), Patient Rehabilitation (Prosthetic limbs and orthotic devices, wheelchairs) and many more.
How do you develop medical devices?
It is essential to ensure that medical devices are safe for use and do not pose significant risks to patients. Evidence-based decision-making can further promote better efficacy and safety based on real world evidence for determining its advantages in terms of safety, efficacy, or cost-effectiveness. Based on the evidence gathered from trials, it provides scope for innovations in diagnostic capabilities, treatment options, and improved patient care. Clinical trials data are an essential element to acquire regulatory approval for the marketing and distribution of medical devices.
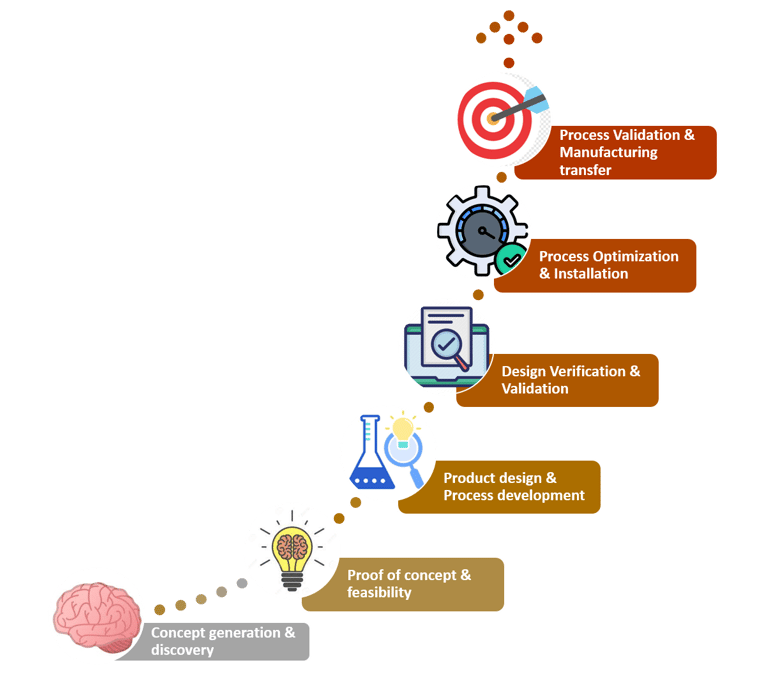
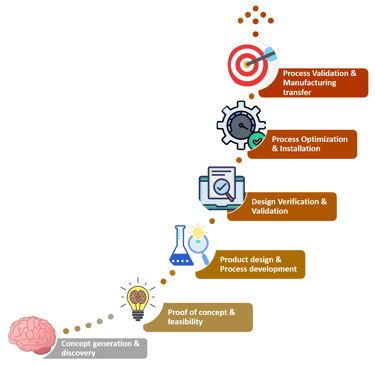
Clinical trials for medical devices, follow a series of phases to assess the safety and effectiveness of the device. Before entering formal clinical trials, medical device developers often conduct feasibility studies and bench testing. These early studies aim to evaluate the device's basic safety, functionality, and potential benefits in a controlled environment. The process further involves cost structuring, competitive analysis, and scalability test. At this stage of conceptualization, the idea is evaluated on multiple grounds to understand its position in the market. It is essential to factor in the geography-specific regulations before finalizing the concept. Once the concept is finalized, it would be strategic to pre-plan for raw materials procurement and logistics to avoid delay in time to market.
Once the supply chain has been set up, the design of the product should be in place. This phase covers Industrial Design, Mechanical Design, Software Design, Electrical Design, and Electronics design. The device must meet the requirements of operation, usability and cost. Followed by designing, is the prototyping stage. It involves multiple iterations of the design to account for the feasibility, scalability and effectiveness of the design created.
Validation is the most important phase to substantiate the device to stand by its market claims. This process begins with preclinical testing which involves testing the medical device in animal models to assess the device's biocompatibility and overall safety. Based on the data, exploratory trials are designed wherein the device is being exposed to a small number of human subjects. Depending on this preliminary data, the device is validated in different study population to assess the device's safety, tolerability, and efficacy. Furthermore, large scale pivotal trials are also inducted to generate substantial evidence for regulatory approvals.
Then comes the phase when all the knowledge about the conceptualized design is transferred to the mass manufacturing stage. Finally, when the product is ready to be launched manufacturers and marketers must be ready to receive and record feedback. Once a detailed analysis of the product feedback has been done necessary changes can be recommended and implemented. After regulatory approval and market release, post-market surveillance should be rigorously executed. This phase involves monitoring the device's performance in real-world clinical settings and collecting data on long-term safety and effectiveness.
What will challenge your trial?
Since the patient population may be suffering from severe form of their indication, recruitment of the study population, consenting and safety can pose as a major challenge. Given the sensitivity and specificity of the medical devices, investors or sponsors often hesitate to fund trials of this nature. Due to variability in algorithms of the medical devices, at times the blinding of the study can be compromised.
To mitigate such, the clinical trials of these nature are run by a variety of highly trained healthcare professionals.
A measure of risk can be estimated by identifying the hazards. Some potential risks can either be mitigated or managed. The responsibilities and accountability should be clearly elucidated. There should be clarity in the criteria of matters that calls for authorization. The responsibility of personnel handling it should be clearly defined. The skills and knowledge necessary to implement the system should be reiterated with a provision for training those who do not possess these skills. One should diligently practice documentation to demonstrate conformance to policies and procedures.
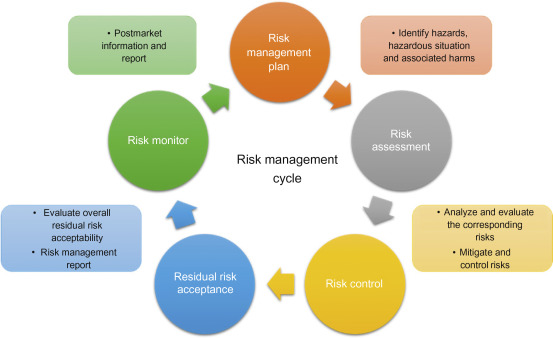
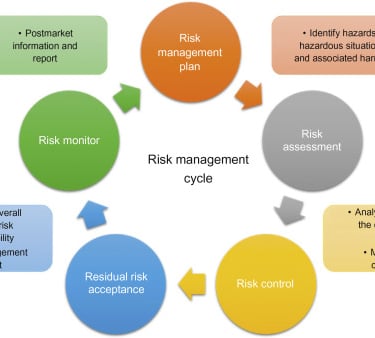
Medical device clinical trials are fundamental to international mandates for product safety. Consequently, it is to ensure that the safety of the general population should always be the highest priority. We at RamAayanaM wish to be a part of your journey in substantiating your medical device in the realms of diagnosis, treatment and rehabilitation. Your Innovation and our regulation will collaborate to provide your devices to the emerging markets and to improve on existing devices.
Leave a Reply
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
