The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137
How to Navigate the Challenges of Clinical Trials in India from Phase-I to Phase-IV
As we are aware that India holds immense potential as a destination for clinical research. With its genetically diverse population, vast patient pool, and cost-effective operational environment, India is already well-positioned to support global drug development. Yet, the journey from early-phase trials to post-marketing surveillance is a bit complex. Despite all the technologies, India's clinical research industry has combatted several disruptions over the past decade. Previous research highlighted a notable dip in early-phase trials followed by regulatory reforms post 2013, which were introduced in response to concerns over ethics and patient safety. While the regulatory changes aimed at strengthening the regulatory framework, they also created temporary setbacks for sponsors and investigators. Restrictions on the number of concurrent trials per investigator, ambiguities in the audio-visual informed consent process, and an uncertain review timeline became key roadblocks. The impact of operational hurdles in clinical research was particularly evident in early-phase research. In this blog we will talk about the challenges which are often associated with each phase of the clinical trial journey in India, and how RCS helps sponsors navigate them efficiently, ethically, and successfully. So, let’s read further.
10/28/20254 min read
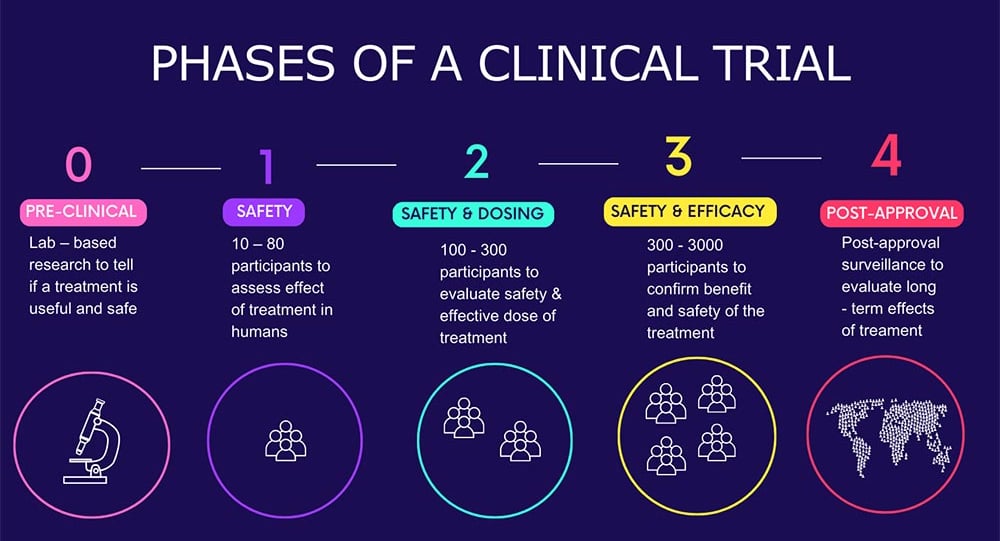
Phase-I: The Foundation of Clinical Research
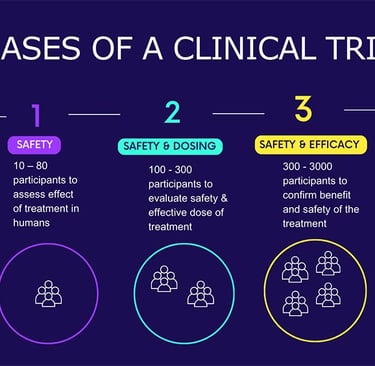
Phase-I trials demand rigorous oversight, ethical sensitivity, and specialized infrastructure. In India, one of the most persistent challenges has been the availability of high-quality Phase-I units equipped with real-time monitoring, emergency response capabilities, and experienced clinical pharmacologists. Recruiting healthy volunteers who meet strict eligibility criteria adds another layer of complexity, especially given the public perception and cultural hesitations around early-phase research.
Furthermore, the DCGI and Ethics Committees subject Phase-I protocols, especially first-in-human and bioequivalence studies to intense scrutiny. Delays in approvals, inconsistent interpretations of safety protocols, and the absence of standardized processes across review boards have, in the past, slowed timelines.
RCS addresses these barriers with a strategic blend of infrastructure and expertise. Our purpose-built Phase-I facilities operate in accordance with international GCP standards, offering continuous medical supervision and seamless data integration. Our experienced regulatory team works closely with the CDSCO, navigating the approval landscape with precision. Additionally, our proprietary volunteer registry enables fast, ethical recruitment with high retention, ensuring both compliance and speed.
Phase-II: When Efficacy Matters
In Phase-II, the objective shifts from safety to efficacy, requiring a balance between scientific rigor and operational flexibility. This is the phase when recruitment becomes more challenging, as patient selection criteria narrow to reflect specific disease conditions and endpoints. Additionally, with Phase II trials (usually spanning multiple sites), ensuring consistency in data capture, patient management, and protocol adherence becomes a pressing concern.
India’s healthcare infrastructure further complicates these trials. Many public hospitals, though rich in patient volume, lack the streamlined administrative processes necessary for clinical trial operations. Private hospitals, on the other hand, may have better resources but face constraints related to cost, ethics committee turnaround times, and investigator bandwidth.
At RCS, we tackle these challenges through data-driven site selection, supported by predictive analytics that analyze historical performance, patient availability, and investigator experience. Our centralized trial management approach ensures protocol compliance across sites, while our real-time data systems including eSource and validated EDC platforms enhance both accuracy and audit readiness. By proactively engaging with investigators and patients alike, we enable smoother recruitment and greater retention, minimizing the risk of mid-trial disruptions.
Overcoming India’s Clinical Research Landscape
As we are aware that India holds immense potential as a destination for clinical research. With its genetically diverse population, vast patient pool, and cost-effective operational environment, India is already well-positioned to support global drug development. Yet, the journey from early-phase trials to post-marketing surveillance is a bit complex.
Despite all the technologies, India's clinical research industry has combatted several disruptions over the past decade. Previous research highlighted a notable dip in early-phase trials followed by regulatory reforms post 2013, which were introduced in response to concerns over ethics and patient safety. While the regulatory changes aimed at strengthening the regulatory framework, they also created temporary setbacks for sponsors and investigators. Restrictions on the number of concurrent trials per investigator, ambiguities in the audio-visual informed consent process, and an uncertain review timeline became key roadblocks. The impact of operational hurdles in clinical research was particularly evident in early-phase research.
In this blog we will talk about the challenges which are often associated with each phase of the clinical trial journey in India, and how RCS helps sponsors navigate them efficiently, ethically, and successfully. So, let’s read further.
Phase-III: The Pivotal Phase
Phase III trials are generally the most complex and resource-intensive stage of clinical development. These large-scale, multi-center studies require coordination across diverse geographies, involving hundreds to thousands of patients. In India, logistical challenges are highly observed along with regulatory delays and inconsistencies in ethics committee reviews. Plus, a major concern during Phase-III execution is patient retention. With long durations and frequent site visits, dropout rates can significantly impact data integrity. Added to this are protocol amendments, which are often necessary but can create operational inefficiencies, especially in decentralized or resource-limited settings.
To navigate these complexities, RCS offers a comprehensive Phase-III trial management model that combines regulatory foresight with operational excellence. Our strong relationships with the DCGI and local ethics committees allow us to anticipate and address potential regulatory hurdles early. We also deploy dedicated site support teams to ensure protocol adherence and build lasting investigator engagement through consistent training, communication, and incentive alignment. Most importantly, our patient-centric solutions, ranging from transportation support to digital engagement tools, make trial participation easier and more accessible, significantly reducing dropout rates.
Phase-IV: Real-World Evidence and Long-Term Safety
While often underestimated, Phase-IV trials are vital for understanding long-term safety, efficacy, and patient behavior in real-world settings. In India, however, the lack of integrated healthcare data systems and variable physician participation have hindered the scope and quality of post-marketing surveillance.
Another major challenge is regulatory compliance. After a substantial reform India’s pharmacovigilance system now requires sponsors to strictly abide by the stringent reporting standards for adverse events and periodic safety updates.
At RCS, our pharmacovigilance division delivers full-spectrum support, from case intake and safety data management to signal detection and regulatory reporting in compliance with both local and global requirements. We develop customized patient registries and leverage digital platforms to collect and analyze real-world data more effectively. By integrating our medical affairs teams into post-marketing strategies, we enhance stakeholder awareness and encourage timely, accurate reporting.
RCS: Your Strategic CRO Partner in India
As the clinical research ecosystem in India matures, the need for agile, expert-driven partnerships becomes increasingly critical. But RCS offers more than just operational support, we provide strategic insight, regulatory expertise, and a deep understanding of the Indian clinical trial landscape.
Our cross-functional teams bring global standards and local experience to every study we undertake, ensuring that timelines are met, data quality is preserved, and ethical standards are upheld. Whether you're launching a first-in-human trial or conducting long-term post-marketing surveillance, RCS is equipped to guide you at every step with the confidence, clarity, and compliance you need to succeed.
If you're planning your next clinical trial, partner with RCS and let us help you turn complexity into progress. For more information connect with us at info@rclinicalsolution.com or visit https://www.rclinicalsolution.com and grab the best available offers.


Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
