The Central Drugs Standard Control Organization (CDSCO) Registered: CRO/MH/2025/000137
Best Practices for Sample Management in Preclinical and Clinical Trials
Sample management plays a vital role in both preclinical and clinical trials because it ensures that every biological sample whether it’s blood, tissue, or cells is handled with accuracy and care from start till end of the study. Managing samples properly in preclinical studies helps researchers avoid contamination or data inconsistencies, all of which could compromise the validity of early safety and efficacy findings. The stakes get even higher once the trials move into the clinical phase and involve human participants. Each sample must be clearly linked to the right person, stored under the correct conditions, and tracked throughout its journey to ensure both scientific accuracy and patient safety. Good sample management also helps teams stay in line with regulatory standards like GLP and GCP, which demand detailed records and traceability. It helps the entire process run smoothly, reducing errors, avoiding delays, and building confidence in the trial’s results. Hence, to protect the sample and data, it is of utmost importance to work on the key principles of comprehensive lab management, and follow best practices in drug discovery, pre-clinical investigations, and human clinical trials.
6/23/20254 min read
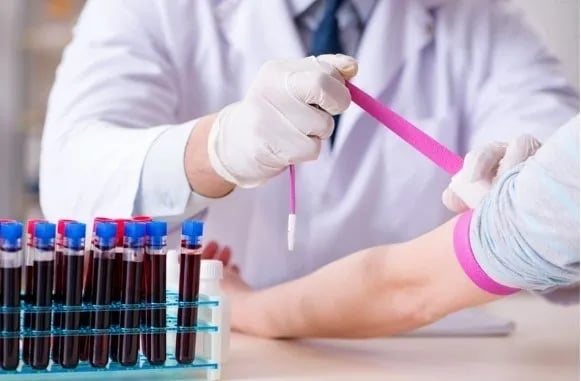
Best Practices for Sample Management in Preclinical and Clinical Trials
Sample management plays a vital role in both preclinical and clinical trials because it ensures that every biological sample whether it’s blood, tissue, or cells is handled with accuracy and care from start till end of the study. Managing samples properly in preclinical studies helps researchers avoid contamination or data inconsistencies, all of which could compromise the validity of early safety and efficacy findings. The stakes get even higher once the trials move into the clinical phase and involve human participants. Each sample must be clearly linked to the right person, stored under the correct conditions, and tracked throughout its journey to ensure both scientific accuracy and patient safety. Good sample management also helps teams stay in line with regulatory standards like GLP and GCP, which demand detailed records and traceability. It helps the entire process run smoothly, reducing errors, avoiding delays, and building confidence in the trial’s results.
Hence, to protect the sample and data, it is of utmost importance to work on the key principles of comprehensive lab management, and follow best practices in drug discovery, pre-clinical investigations, and human clinical trials.
Following are some of the best measures to undertake while managing samples in trials:
Establishing and Maintaining Standard Operating Procedures (SOPs)
Standardized workflows are essential to ensure sample consistency and reduce variability. SOPs should be properly prepared and maintained to govern every step of the sample management including acquisition, processing, storage, transfer, and disposal. Clear documentation and frequent training promote adherence, while regular reviews of SOPs help integrate evolving regulatory and technical requirements.
Integrating Sample Tracking with Digital Infrastructure
Digital platforms such as Laboratory Information Management Systems (LIMS) or biorepository management software are key factors. These systems support real-time tracking, provide audit trails, and facilitate sample annotation with associated metadata. Integration with electronic lab notebooks (ELNs) and clinical trial management systems (CTMS) ensures that the sample is traceable across both the lab and patient-level data.
Sample Identification and Feasible Storage Conditions
Easy and quick sample identification is pivotal for cross-study comparability and data integration. To make the process efficient, barcoding and RFID tagging along with metadata capture including subject ID, collection date, storage conditions, etc., support the process ahead and reduce the risk of mislabeling. Biological sample integrity is highly sensitive to temperature fluctuations and humidity. The research facilities should make sure that validated cold storage logistics equipped with continuous monitoring system is being used for sample storage and transit. This helps in prevention of sample degradation.
Maintaining a Transparency and Multidisciplinary Coordination
Every interaction with a sample should be logged, whether it involves transport, thawing, aliquoting, or analytical testing. Digitized logs should be used which help track sample status and enable rapid response to discrepancies or audits. In addition to that, regular communication between clinical operations and laboratory staff ensures alignment on protocols and timelines. This communication helps in the timely and effective usage of samples thereby avoiding damage, duplication, and improve resolution of sample-related queries.
Proper Quality Control and Planning for Risk and Contingency
Routine quality control assessments such as temperature checks, sample viability testing, and system validation help detect and mitigate issues at an early phase. Good quality assurance measures strengthen data reliability and regulatory awareness. Besides, addressing the possible risks with proper strategies is crucial; risks such as power failures, shipping delays, and equipment malfunctions should be addressed by mitigation strategies which may include duplicate sample storage at various locations, validated shipping containers, and SOPs for emergency response. Keeping oneself prepared to handle such issues reduces sample loss during unforeseen events.
Ensuring Regulatory Compliance and Documentation Integrity
Compliance with international regulations such as ICH-GCP, GLP, and FDA is non-negotiable. Maintaining accurate and complete documentation, including electronic trial master files (eTMFs) and sample transfer agreements, is very important during inspections and audits. Thus, it is crucial to conduct regular internal reviews which help ensure that documentation reflects actual practice.
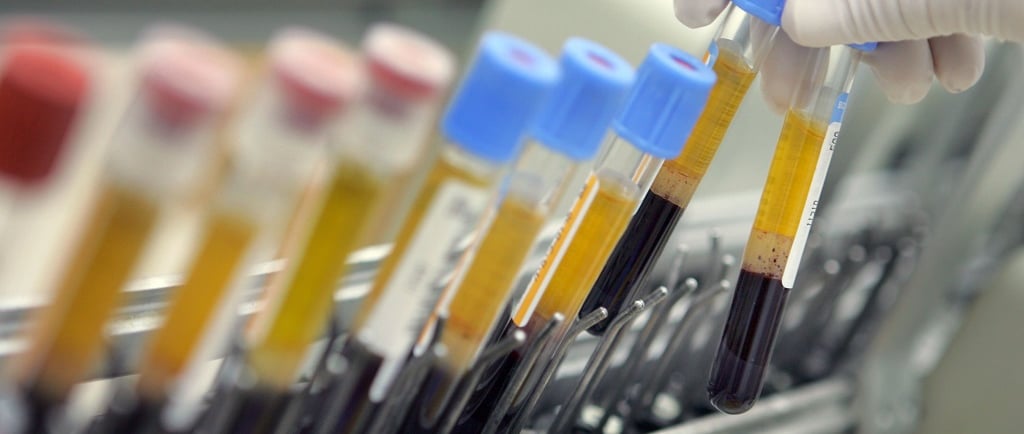
The Role of RCS in Leveraging Sample Management in Trials
In the complex environment of clinical and preclinical research, working with a specialized partner like RCS can significantly enhance sample management capabilities. With extensive domain expertise, validated infrastructure, and global logistics networks, RCS offers scalable solutions that align with sponsor protocols and regulatory expectations.
Key Benefits:
Centralized Biorepository Services: Secure and controlled storage with excellent monitoring and validated backup systems for both short-term and long-term biological sample preservation.
End-to-End Chain of Custody Tracking: Digitized tracking solutions ensure complete traceability from collection to final disposition, reducing regulatory risk and data discrepancies.
Customizable Data Integration: Tailored data systems allow seamless integration with sponsor platforms, improving visibility, reporting, and cross-functional collaboration.
Regulatory-Grade Documentation Support: RCS provides robust quality assurance systems, audit readiness, and standardized documentation aligned with GLP/GCP requirements.
Expertise in Global Logistics and Compliance: With experience managing cold chain logistics across multiple geographies, RCS ensures timely and compliant transport of clinical specimens and research materials.
By outsourcing sample management to a trusted CRO like RCS, research sponsors can focus their internal resources on core scientific innovation while maintaining confidence in the integrity and security of their biological samples. For more information, please visit our website or contact us at +17373134316.
Subscribe to our newsletter
Quick Links
Socials
Copyright © 2025. RCS - RamAayanaM Clinical Solution. All Rights Reserved
Registered office
Diva East, Thane, Maharashtra, India
Miami, FL, USA
Branch office
Vikhroli West, Mumbai, Maharashtra, India
Vila Franca de Xira, Portugal
Madrid, Spain
Contact us:
+91-8979335208
+91-9820507220
E-mail:
info@rclinicalsolution.com
bd@rclinicalsolution.com
